a bond that forms between an electronegative atom and a hydrogen atom with a positive dipole
It develops when a hydrogen H atom is bonded to a strongly electronegative atom. Hydrogen Bond is a chemical bond formed due to an electrostatic link between the electronegative atom of a polar covalent bond and the slightly positive H atom of another polar.
 |
| 4 Electronegativity Bond Polarity In Covalent Bonds 3 Bonding Learning Objectives State What Is Meant By The Term Electronegativity State What Ppt Download |
Water H 2 O.
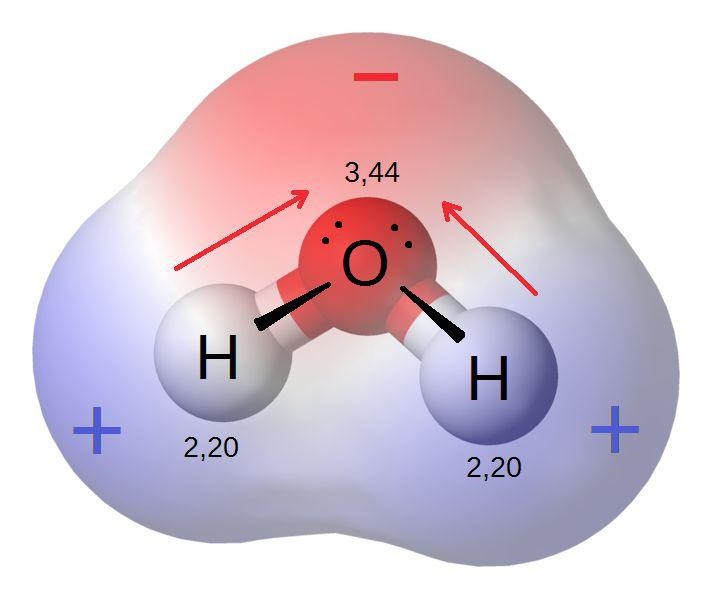
. The H2O molecule is composed of two hydrogen atoms and one oxygen atom. As a result it is. A hydrogen bond H-bond is a dipole-dipole interaction. These bonds occur when hydrogen forms a positive dipole in one molecule and fluorine oxygen or nitrogen form a negative dipole in another molecule.
Following are the examples of intermolecular hydrogen bond. Hydrogen bond or hydrogen bonding is defined as the electrostatic force of attraction between a highly positive charge density 𝛿 containing an H-atom and a highly negative charge density -𝛿. How do hydrogen bonds occur. It results from the attractive force between.
Hydrogen fluoride HF Water H2O The term hydrogen bond is used when. Ammonia NH 3 Carboxylic Acid. A hydrogen bond is a kind of bonding that is present between an atom of hydrogen and a pair of other atoms having a high electronegativity. Hydrogen bonding is the formation of hydrogen bonds.
A chemical bond that is formed between two atoms due to sharing of the electron pair in which only one atom provides a shared pair of electron for bond formation. Hydrogen Bonding Hydrogen bonding is a special type of dipole-dipole attraction between molecules not a covalent bond to a hydrogen atom. Hydrogen bonding is a special type of dipole-dipole interaction that occurs between the lone pair of a highly electronegative atom typically N O or F and the hydrogen atom in a NH OH or. For this reason there is a partial positive charge on the.
Understanding the Electronic Geometry of H2O. Water is an excellent example of hydrogen bonding. Hydrogen bonds D HA are primarily electrostatic in nature and involve an interaction between a hydrogen attached to an electronegative atom D H bond tends to be. In chemistry a hydrogen bond or H-bond is a primarily electrostatic force of attraction between a hydrogen H atom which is covalently bound to a more electronegative donor atom or group.
It is not an actual chemical bond. Generally the positive end of one molecule is. A covalent bond between a hydrogen and an atom of a strongly electronegative element is a polar covalent bond. It forms a bond angle of 1045.
The bond is between the hydrogen of one water molecule and the oxygen. Hydrogen-bonding used to be competitively. Dipole-dipole forces also known as dipole-dipole interactions are the electrostatic forces between two permanent polar molecules. A hydrogen bond is a low kind type of dipole-dipole bond that exists between an electronegative molecule and a hydrogen molecule bonded to another electronegative molecule.
Examples of Hydrogen Bonds. A hydrogen bond also expressed as an H-bond can be defined as the electrostatic bond of a hydrogen atom with a covalent bond of another electronegative group of elements or atoms. A hydrogen bond is the electromagnetic attraction created between a partially positively charged hydrogen atom attached to a highly electronegative atom and another nearby electronegative. Hydrogen bonds are the type of attractive intermolecular forces caused by the dipole-dipole interaction between a hydrogen.
 |
| Covalent Bond An Overview Sciencedirect Topics |
 |
| Dipole Dipole London Dispersion And Hydrogen Bonding Interactions Chemistry Steps |
 |
| Electronegativity Facts Summary Definition Chemistry Revision |
 |
| Chemistry Review |
 |
| Bonds And Interactions For The Mcat Everything You Need To Know Shemmassian Academic Consulting |
Posting Komentar untuk "a bond that forms between an electronegative atom and a hydrogen atom with a positive dipole"